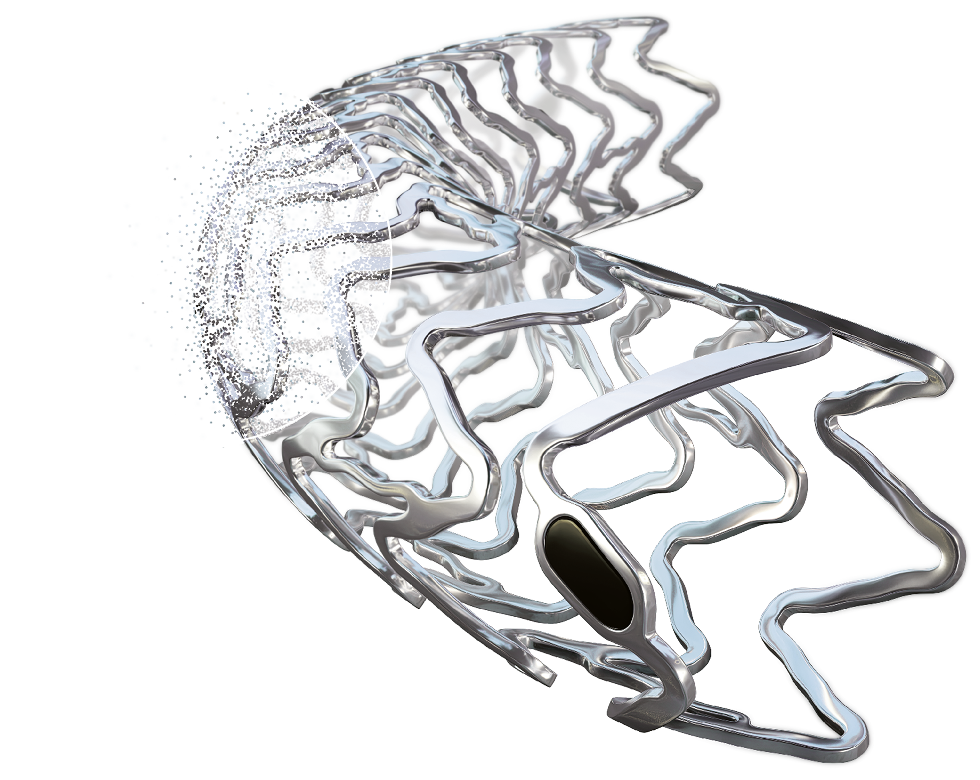
Freesolve™
Resorbable Magnesium Scaffold
Metallic performance1-3.
Fully resorbablea,4.
Download the Brochure
Thank you for your interest in Freesolve™ RMS. Please provide your contact details to download the product brochure.
Key Benefits
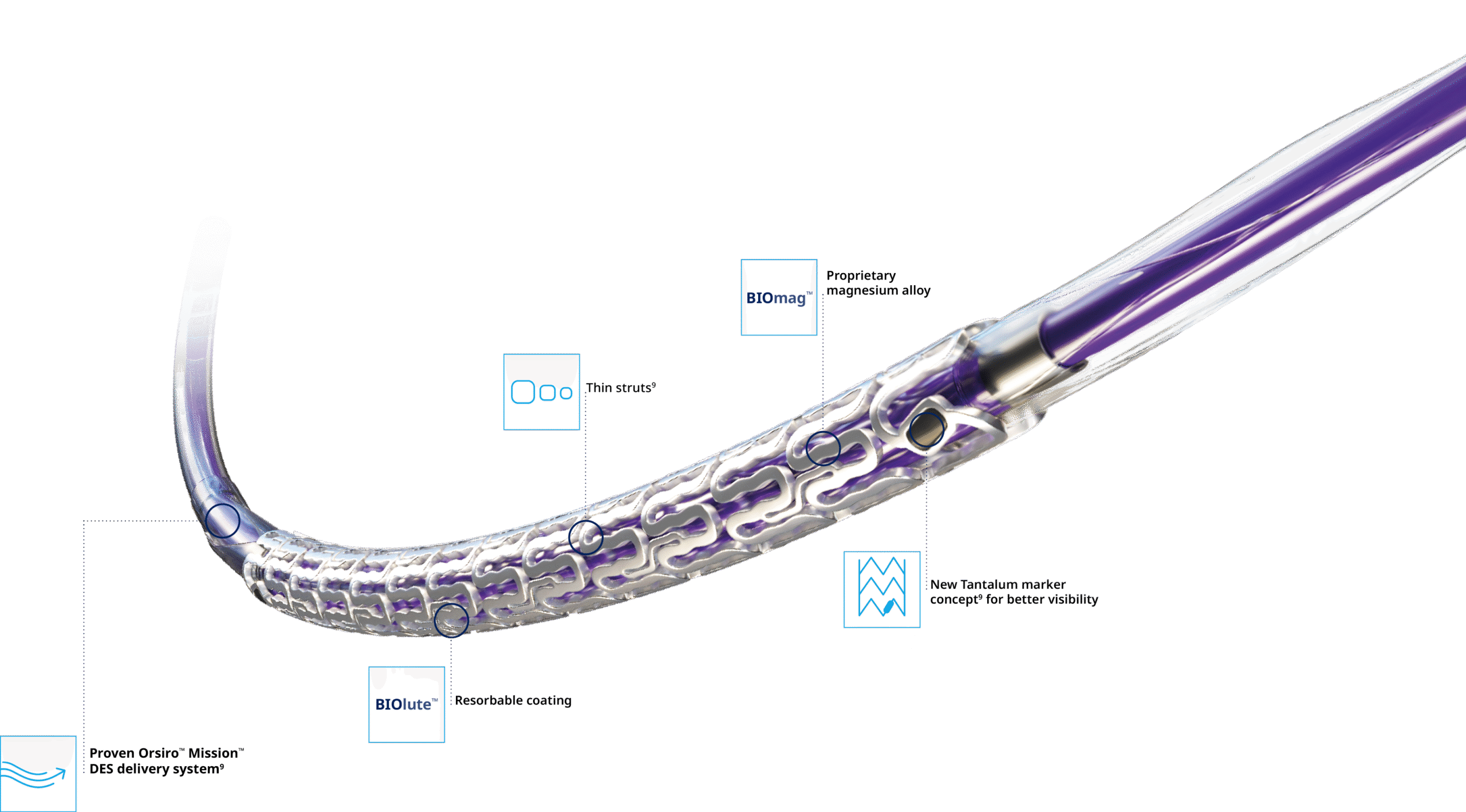
Delivers like a DES5
Optimal vessel support6,7
Magnesium fully resorbed after 12 months8
Excellent safety and efficacy2,3
Full System
Pre-procedure
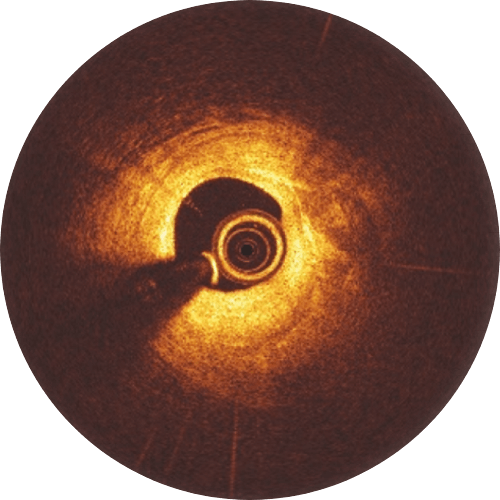
Initial diagnostic
Post-procedure

Immediately after Implantation, struts are well apposed to the vessels wall.
6-month follow-up
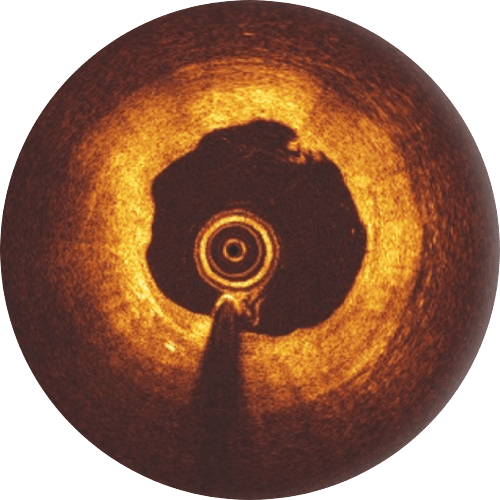
While the Magnesium resorption process continues, endothelialization progresses.
12-month follow-up
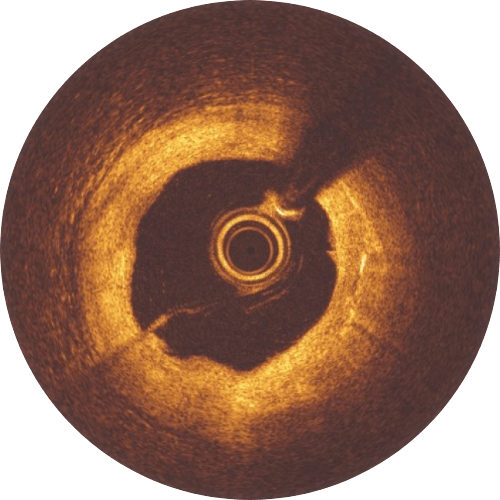
The Magnesium resorption is completed. No struts appear in OCT.
>99%
of struts no longer visible at 12 months8
Magnesium fully resorbed after 12 months8
Procedure
Experience shows that following 4 Ps for Freesolve™ RMS implantations may lead to better patient outcomes.
Patient Selection
Appropriate patient selection is crucial to achieve procedural success. Freesolve™ is currently indicated for de novo lesions, with a reference vessel diameter and lesion length closely matching the available Freesolve™ sizes. Each individual patient should receive best clinical care and should benefit from BRS Technology.
Proper Sizing
If uncertain about the vessel diameter, use QCA, IVUS and/or OCT for quantitative lesion evaluation. The diameters available are 2.5, 3.0, 3.5 and 4.0 mm, do not implant into vessels 4.2 mm in diameter. Angiogram generally underestimates the diameter of the vessel by 0.25 mm.

Pre-Dilatation
Pre-dilatation with a non-compliant balloon with a 1:1 balloon-to-artery ratio is mandatory. The balloon should expand fully. The residual stenosis before the Freesolve™ implantation is recommended to be less than 20 %. If the pre-dilatation goal is not achieved, use other balloon technologies such as scoring balloons.
Post-Dilatation
Post-dilatation with a non-compliant balloon 0.5 mm larger than the implanted scaffold expanded at high pressure (> 16 atm) is recommended. Please keep in mind that the Freesolve™ expansion limit is 0.6 mm beyond nominal scaffold size. During the learning phase, OCT is helpful to check for vessel and lumen dimensions, lesion length and struts’ malapposition.
Clinical Highlights
A wealth of clinical evidence
| Study name | Study type | Patients in clinical study | Test model in pre-clinical study | Status | Endpoint | Flyer (where available) |
|---|---|---|---|---|---|---|
| BIOMAG™-II | RCT | 1,859 | - | Initiated | TLF at 12 months | |
| RMS Registry | Registry | 1,106 | - | Initiated | TLF at 12 months | |
| BIOMAG™-I | FIH | 115 | - | 36m FUP available | In-scaffold LLL at 6 months | Download |
| BIOMAG™-I OCT Analysis* | FIH | 69 | - | 12m FUP available | Visible struts at 6 and 12 months | |
| BIOMAG™-I Return of vasomotion* | FIH | 14 | - | - | Vasomotion assessment after Acetylcholine (Ach) and Nitroglycerine (NTG) | |
| BIOMAG™-I OCT Plaque characterization (AI-based OCT)* | FIH | 84/6m 46/12m | - | - | Comparison of different plaque characteristics | Download |
| BIOMAG™-I Plaque characterization* | FIH | 84 | - | - | Impact of plaque characteristics on in-stent LLL | |
| BIOSOLVE-IV Full COHORT conducted with Magmaris | Registry | 2,066 | - | 36m FUP available | Target Lesion Failure* at 12 months | |
| Evaluation of the degradation kinetics* | Animal study | - | mini swine | Completed | Degradation kinetics analysis: 99.6% backbone degradation for Freesolve™ at 12 months | |
| Evaluation of Thrombogenicity Using Human Blood | In-vitro study | - | flow chamber study | Completed | BIOmag™ scaffold material shows strongly reduced in vitro thrombogenicity | |
| Preclinical animal safety and device characterization up to 4y | Animal study | - | mini swine | Ongoing | Safety evaluation | |
| Chronic Scaffolding Evaluation for DREAMS 3G | In Silicon study | - | simulation | Ongoing | Resorption and Finite element analysis |
*restricted access, sign-in required
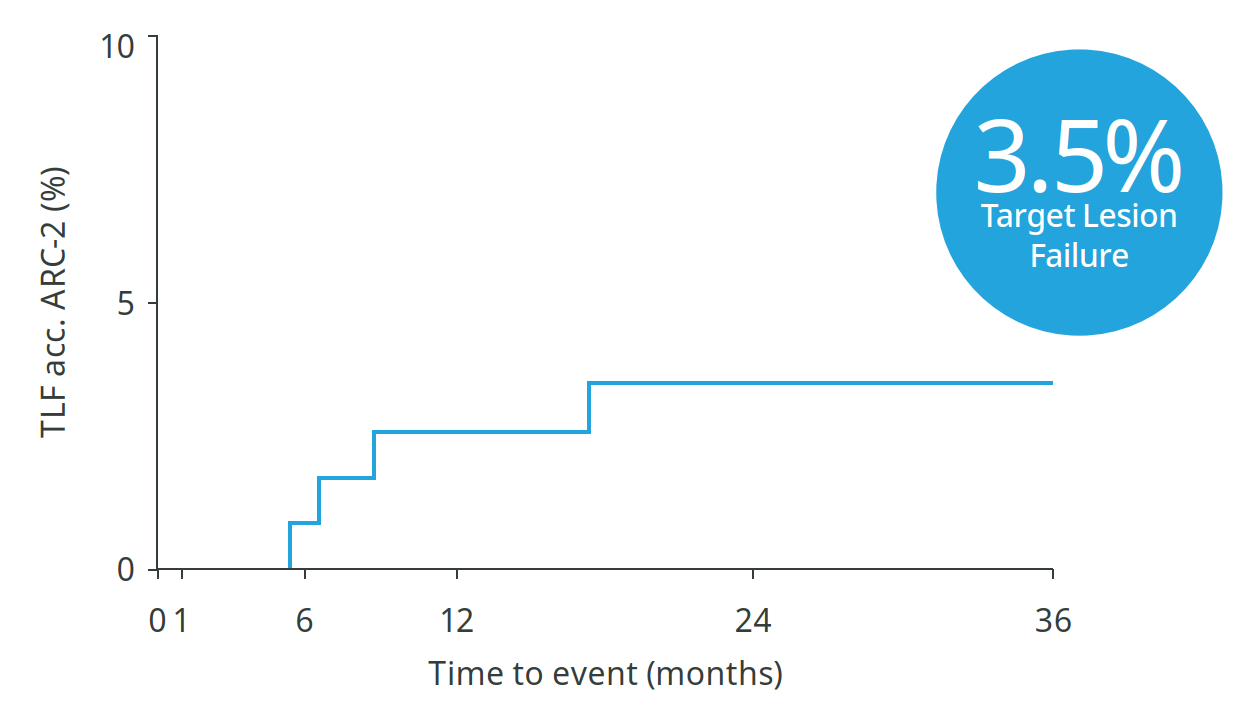
36-month outcomes confirm: No new events between 2 and 3 years9
3.5%
Target Lesion Failure
Scaffold Thrombosis
Myocardial Infarction
Cardiac Death
CAUTION—Investigational device. Limited by the United States law to investigational use.
Freesolve is clinically often referred to as DREAMS 3G.
a. 99.3% resorbed at 12 months (markers are not resorbable), based on clinical data.
1. Data on file; benchtest data; 2. Haude M. et al., the Lancet eClinicalMedicine 2023;59: 101940; 3. Haude, M. et al., EuroIntervention 2023;19:1-1 published online May 2023;
4. Seguchi M et al. OCT Analysis 12M, presented at ESC 2023; 5. Data on file, Freesolve in comparison to Orsiro Mission and Abbott Xience Sierra;
6. Based on pre-clinical data, Seguchi, M. et al., EuroIntervention 2023;18-online publish-ahead-of-print January 2023; 7. Data on file, in comparison to predecessor device;
8. Based on intravascular OCT analysis of the BIOMAG-I trial presented by Dr. M. Seguchi at ESC 2023; 9. Data on file; 10. Haude, M. et al., Clinical outcomes of the third-generation resorbable magnesium scaffold for coronary artery lesions: three-year results of the BIOMAG-I study. EuroIntervention 2025;21:e1-e3.
BIOSOLVE-II and BIOMAG-I based on Kaplan-Meier failure estimate analysis.
Teleflex, the Teleflex logo, BIOMAG, BIOmag, BIOlute, Freesolve, Magmaris, Orsiro, and Orsiro Mission are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. All other names are the trademarks or registered trademarks of their respective owners. Information in this material is not a substitute for the product Instructions for Use. Not all products may be available in all countries. Please contact your local representative. Revised 09/2025. ©2025 Teleflex Incorporated. All rights reserved.