Passeo™-18 Lux™
Drug-Coated Balloon Catheter
Treat long.
Achieve more with less
Now available in 150 mm and 200 mm

Download the Brochure
Thank you for your interest in Passeo™-18 Lux™ DCB. Please provide your contact details to download the product brochure.
Key Benefits
Clinically proven
Safe and effective in the treatment of lower limb arteries1,2
For challenging patients
Excellent results despite a complex population at baseline3
Effective drug delivery
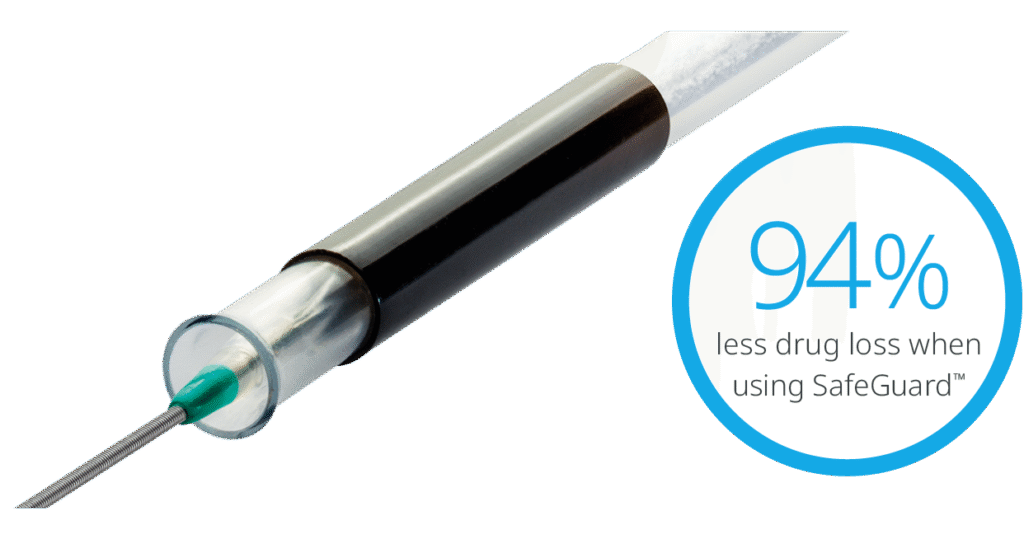
Reduction of drug loss with SafeGuard™ Insertion Aid4
Clinically proven
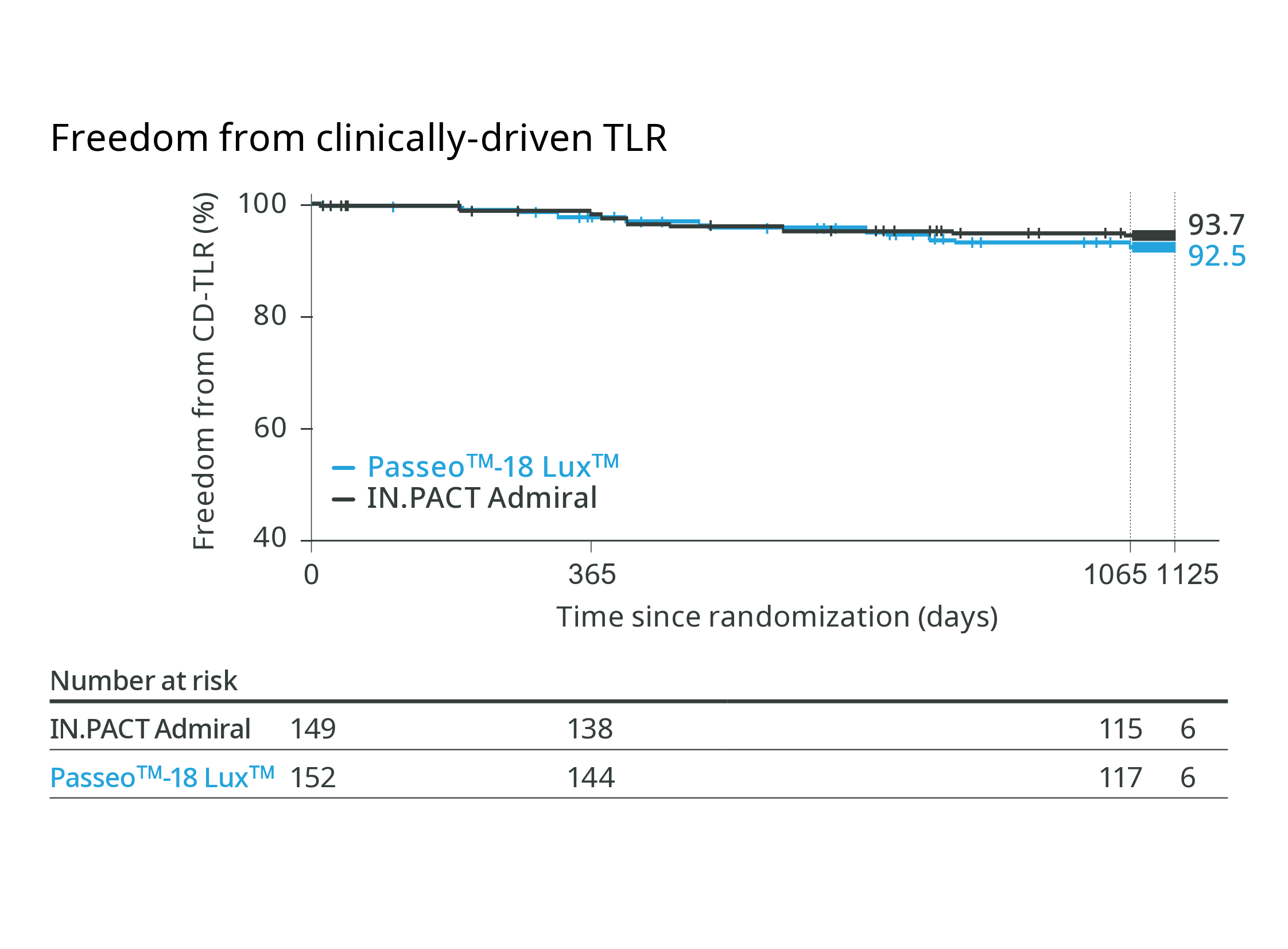
BIOPACT RCT
Passeo™-18 Lux™ DCB has shown non-inferiority to the IN.PACT Admiral DCB at follow-up5,6,7
36-month outcomes
92.5% (Passeo™-18 Lux™) vs. 93.7% (IN. PACT Admiral)
Freedom from cd-TLR
92.5% (Passeo™-18 Lux™) vs. 93.7% (IN. PACT Admiral)
Freedom from major target limb amputations
BIOLUX P-III
All-comers 24-month outcomes confirm Passeo™-18 Lux™ DCB’s safety and effectiveness in infra-inguinal arteries3
24-month outcomes
88.1%
Freedom from cd-TLR
96.5%
Freedom from major amputations
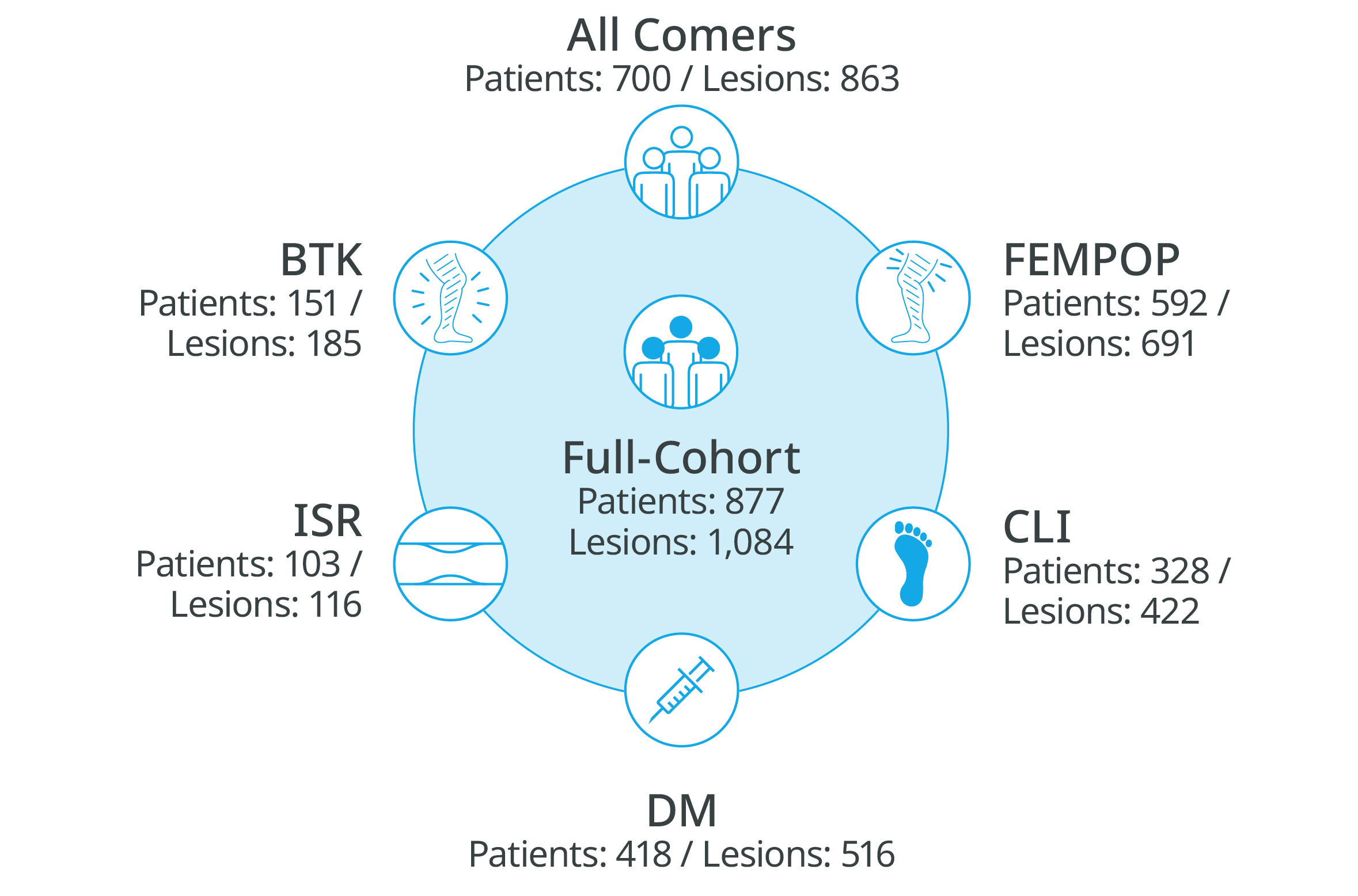
For challenging patients
Safety and efficacy clinically proven across challenging subgroups in BIOLUX P-III all-comers registry
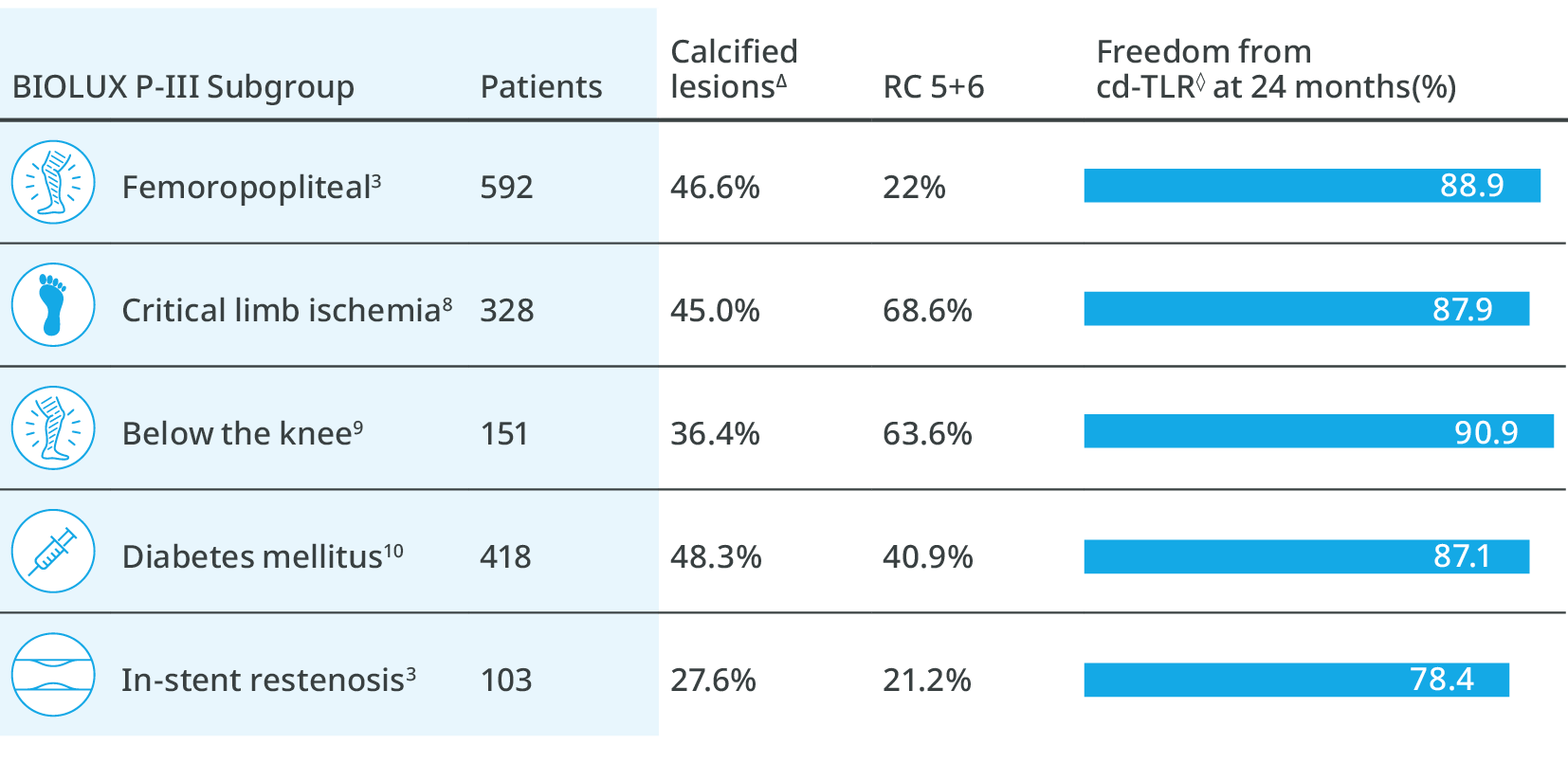
Freedom from major target limb amputation at 24 months
93.9%
CLI subgroup
90.1%
BTK subgroup
Effective drug delivery
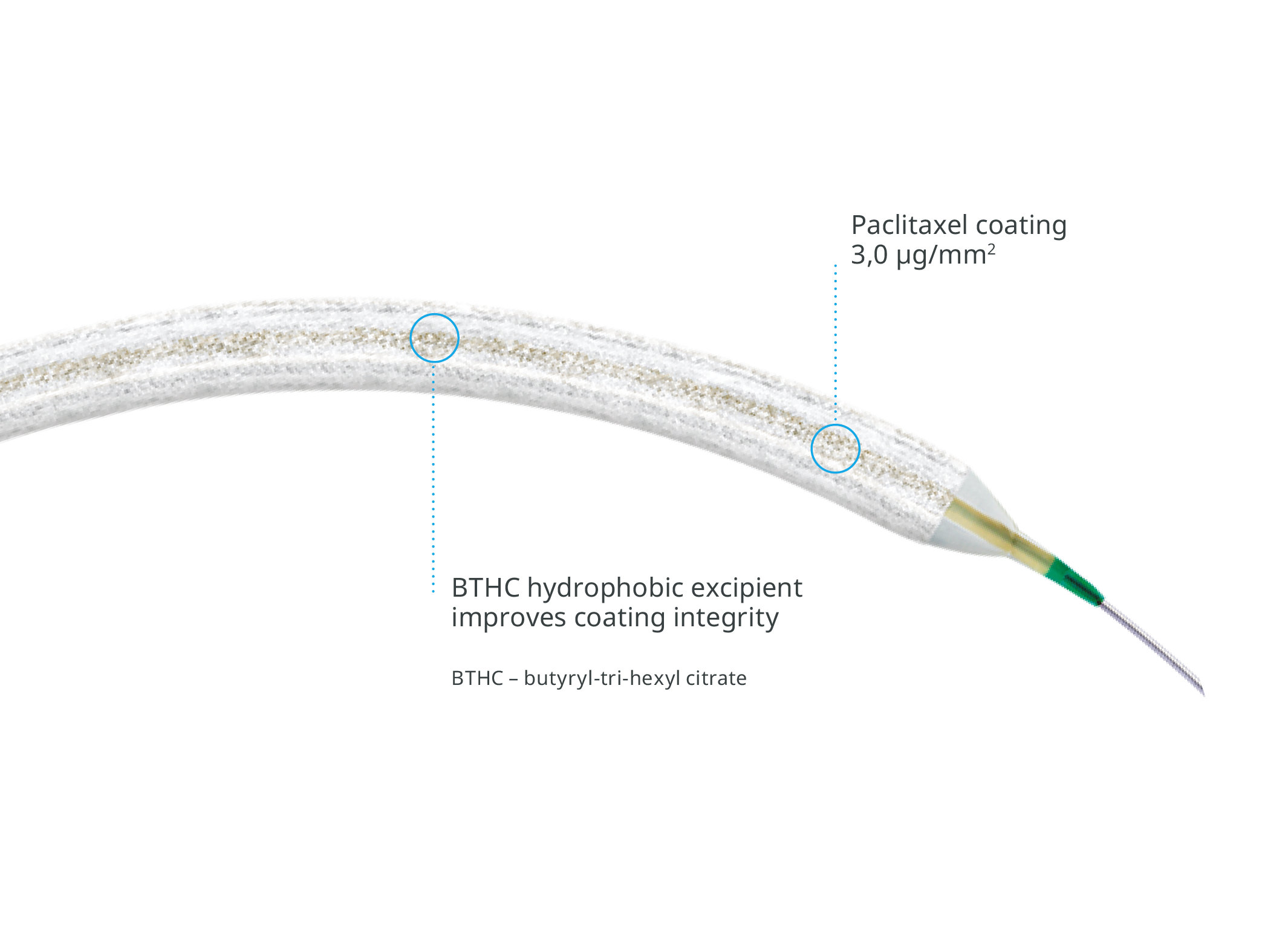
LUX™ COATING
High drug retention coating with hydrophobic BTHC excipient11

Drug coating integrity: % of drug load remaining on balloon after being submerged for ~90 seconds in physiological solution.
Insertion and handling
The SafeGuard™ insertion aid improves ease of handling, and protects the user and balloon coating from contact and damage.
Passeo™-18 Lux™ in Action
REACT strategy: Treating Long SFA Occlusions with a Single Long-Length Drug-Coated Balloon (DCB) + Bare-Metal Stent (BMS)

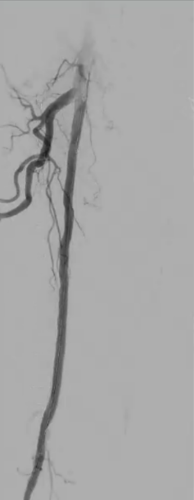
Patient history
An 81-year-old woman presented with claudication of the right leg.
Procedure summary
Angiography showed a flush occlusion at the bifurcation between the deep femoral artery (DFA) and superficial femoral artery (SFA). After crossing, control angiography showed a diffuse, sub-occlusive long SFA lesion (Figure 1).
Pre-dilatation was performed with plain angioplasty balloon. Next, one long-length Paclitaxel-coated Passeo™-18 Lux™ DCB (6 x 200 mm) was inflated for three minutes to treat the segment, followed by a Pulsar™-18 T3 BMS.
Results
The final angiography showed complete revascularization of the femoropopliteal segment (Figure 2).
Drug-Coated Balloon Treatment for Critical Limb Ischemia

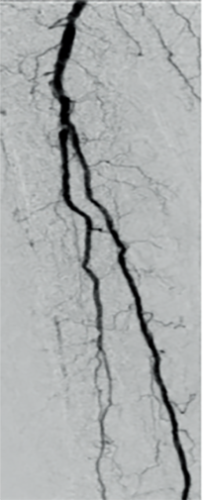
Patient history
71-year-old man admitted due to critical ischemia of the right lower limb, with painful and non-healing necrosis of the first and second fingers. Diagnosis showed total occlusion of the tibial and dorsal arteries on the right side (Figure 1).
Procedure summary
Angioplasty was performed for 3 minutes in the right posterior tibial artery, in the tibiofibular trunk and in the fibular artery. Angioplasty of the distal PTA and lateral plantar artery was performed with Passeo™-18 Lux™ DCB.
Results
Completion angiography demonstrated effective recanalization after use of Passeo™-18 Lux™ DCB, with normal flow to the plantar arch (Figure 2).
Improving Potential Long-Term Patency of Common Femoral Artery Stenoses
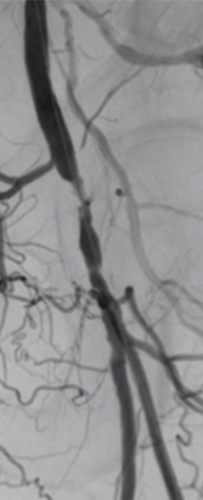
Patient history
66-year-old male presented with impaired walking capacity of the left leg. Angiography confirmed significant stenosis of the left common femoral artery (Figure 1).
Procedure summary
Angiography showed a flush occlusion at the bifurcation between the deep femoral artery (DFA) and superficial femoral artery (SFA). After crossing, control angiography showed a diffuse, sub-occlusive long SFA lesion (Figure 1).
Pre-dilatation was performed with plain angioplasty balloon. Next, one long-length Paclitaxel-coated Passeo™-18 Lux™ DCB (6 x 200 mm) was inflated for three minutes to treat the segment, followed by a Pulsar™-18 T3 BMS.
Results
The final angiography showed complete revascularization of the femoropopliteal segment (Figure 2).
REACT strategy: DCB + BMS for a Long SFA Occlusion

Patient history
79-year-old male presented with severe claudication and intermittent rest pain of the right leg. Angiography showed a long occlusion of the SFA (Figure 1).
Procedure summary
Predilatation performed with plain angioplasty balloon. Next, three Passeo™-18 Lux™ DCB were used to cover the entire occluded segment, followed by a Pulsar™-18 T3 Bare-Metal Stent.
Results
Final angiography showed complete revascularization of the femoropopliteal segment without dissection and fast runoff to the foot (Figure 2).
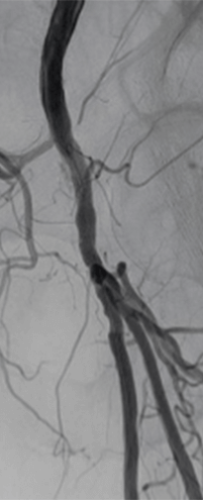
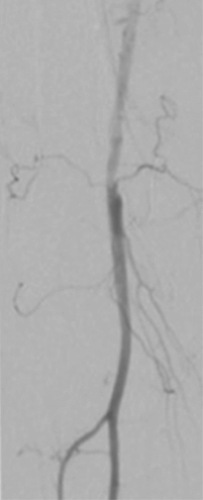
Distal Popliteal Access for Low-Profile Stenting and DCB Therapy
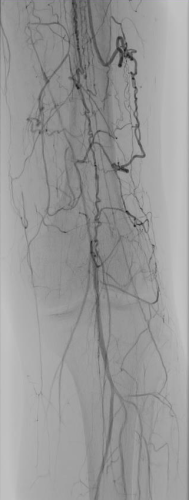
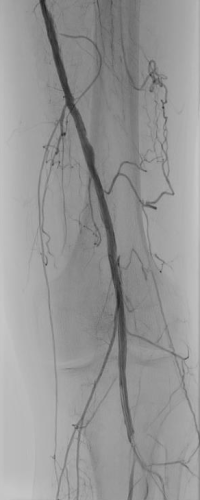
Patient history
A 62-year-old patient presented with a history of multiple interventions on both legs, including amputation and a bypass. Angiography showed an occlusion of the superficial femoral artery (Figure 1).
Procedure summary
Retrograde challenging access and crossing with extravasation. A 4F low-profile Pulsar™-18 T3 stent was placed at the ostium of the SFA, distal SFA and popliteal arteries were then treated with Passeo™-18 Lux™ DCB.
Results
Patent SFA with strong outflow into the tibial arteries and a good blood supply into the foot (Figure 2).
Links to studies
- BIOPACT RCT 3-year (link: https://pubmed.ncbi.nlm.nih.gov/41432343/)
- Peeters M, et al. J Cardiovasc Surg (Torino). 2025;66(6):557-565.
- BIOPACT RCT 1-year (link: https://pubmed.ncbi.nlm.nih.gov/38092496/)
- Deloose KR, et al. JACC Cardiovasc Interv. 2023;16(23):2900-2914.
- BIOLUX P-III Full Cohort 2-year (link: https://pubmed.ncbi.nlm.nih.gov/33083853/)
- Tepe G, et al. Cardiovasc Intervent Radiol. 2021;44(2):207-217.
- BIOLUX P-III Below-the-Knee 2-year (link: https://pubmed.ncbi.nlm.nih.gov/32964317/)
- Tepe G, et al. Cardiovasc Intervent Radiol. 2021;44(1):10-18.
- BIOLUX P-III Critical Limb Ischemia 2-year (link: https://pubmed.ncbi.nlm.nih.gov/32950415/)
- Brodmann M, et al. JACC Cardiovasc Interv. 2020;13(19):2289-2299.
- BIOLUX P-III Long-Segment Femoropopliteal 1-year (link: https://pubmed.ncbi.nlm.nih.gov/37422253/)
- Angel de Gregorio M, et al. J Vasc Interv Radiol. 2023;34(10):1707-1715.e7.
- BIOLUX P-III Full Cohort 1-year (link: https://pubmed.ncbi.nlm.nih.gov/31989855/)
- Tepe G, et al. J Endovasc Ther. 2020;27(2):304-315.
- BIOLUX P-II 1-year (link: https://pubmed.ncbi.nlm.nih.gov/26493253/)
- Zeller T, et al. JACC Cardiovasc Interv. 2015;8(12):1614-1622.
- BIOLUX P-I 1-year (link: https://pubmed.ncbi.nlm.nih.gov/25775674/)
- Scheinert D, et al. J Endovasc Ther. 2015;22(1):14-21.
References:
- Scheinert D. PTX Releasing Balloon in femoropopliteal lesions using BTHC excipient: 12-month results from the BIOLUX P-I randomized trial. JEVT, 2015; 22(1): 14-21;
- Zeller et al. PTX-Coated Balloon in Infrapopliteal arteries 12-month results from the BIOLUX P-II randomized trial. J Am Coll Cardiol Intv. 2015; 8: 1614-22;
- Tepe G. PTX-Coated Balloon Angioplasty for the Treatment of Infrainguinal Arteries: 24-Month Outcomes in the Full Cohort of BIOLUX P-III Global Registry. Cardiovasc Intervent Radiol.2021;44:207-217;
- Data on file;
- Deloose K. The head-to-head Passeo-18 Lux vs IN.PACT Admiral BIOPACT RCT: latest release of the 36 month outcomes. Presented at: LINC 2025; January 28-30, 2025; Leipzig, Germany;
- Deloose KR, Lansink W, Brodmann M, et al. Head-to-Head Comparison of 2 Paclitaxel-Coated Balloons for Femoropopliteal Lesions. JACC: Cardiovascular Interventions 2023;16:2900-14;
- Data on file;
- Brodmann M et al. Real-World Experience With a PTX-Coated Balloon in Critical Limb Ischemia 24-Month Subgroup Outcomes of BIOLUX P-III. JACC Cardiovasc Interv. 2020;13:2289-2299;
- Tepe G et al. BIOLUX P-III Passeo-18 Lux All-Comers Registry: 24-Month Results in Below- the-Knee Arteries. Cardiovasc Intervent Radiol. 2021;44:10-18;
- Mwipatayi P, Barry I, Brodmann M, et al. Twenty- Four-Month Outcomes of Drug-Coated Balloon in Diabetic Patients in the BIOLUX P-III Registry: A Subgroup Analysis. Annals of Vascular Surgery (2021); https://doi.org/10.1016/j.avsg.2021.02.050;
- Data on file.
The Passeo-18 Lux DCB with its Lux coating is part of the Lux family of Paclitaxel-coated balloons.
Teleflex, the Teleflex logo, Lux, Passeo, Pulsar and SafeGuard are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. All other names are the trademarks or registered trademarks of their respective owners. Refer to this IFU link for a copy of the Instructions for Use and for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use. Not all products may be available in all countries. Please contact your local representative. Revised 09/2025. ©2025 Teleflex Incorporated. All rights reserved.
Images for illustration purposes only, may not be an exact representation of the device.