Dynetic™-35
Balloon-Expandable Cobalt Chromium Stent System
The next generation iliac stent with excellent radial strength and superb flexibility.1,2
Download the Brochure
Thank you for your interest in Dynetic™-35 stent. Please provide your contact details to download the product brochure.
Key Benefits
High radial strength2
Thin struts, super flexible1 Cobalt Chromium stent design
Largest size range3
Complete size range with diameters of 5.0-10.0mm and lengths of 18-78mm
Deliverability in 6F compatibility4
Low profile balloon catheter with excellent deliverability2
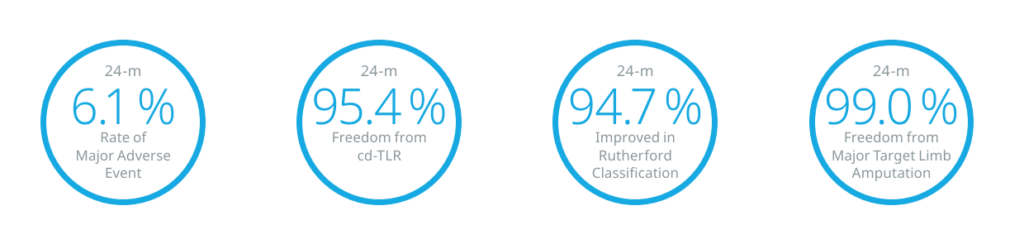
BIONETIC-I
Prospective, international, multicenter, single-arm study with up to 60-month follow-up evaluating the safety and efficacy of Dynetic™-35 in the treatment of peripheral artery disease in the iliac arteries.
N = 160 subjects
Country: Austria, Belgium, France, Germany, Hungary, Latvia
Primary Endpoint: Major adverse events§ 12 months post-index procedure
§Major adverse events includes device or procedure related death within 30 days post index procedure, clinically driven target lesion revascularization,
and major index limb amputation up to 12 months post index procedure.
High radial strength2 and 6F compatible deliverability across the largest size range.3,4
Thin struts with high radial strength
High radial strength
Higher radial strength compared to leading competitor Cobalt Chromium (CoCr) stents.2
Super flexible stent design
Most flexible stent design compared to leading competitor stents.1
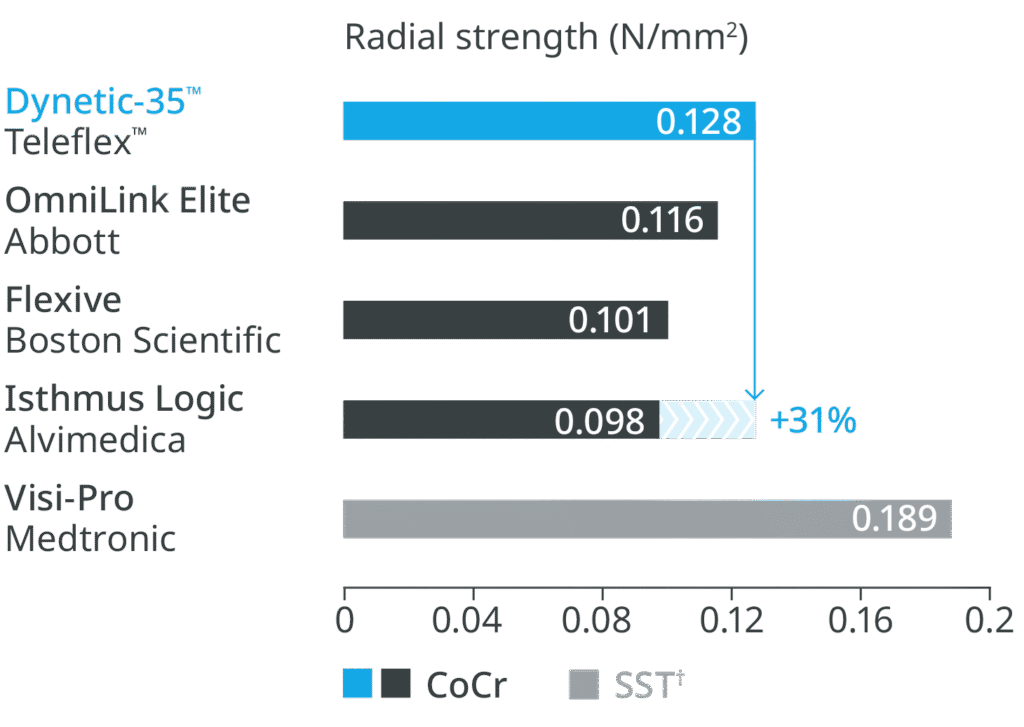
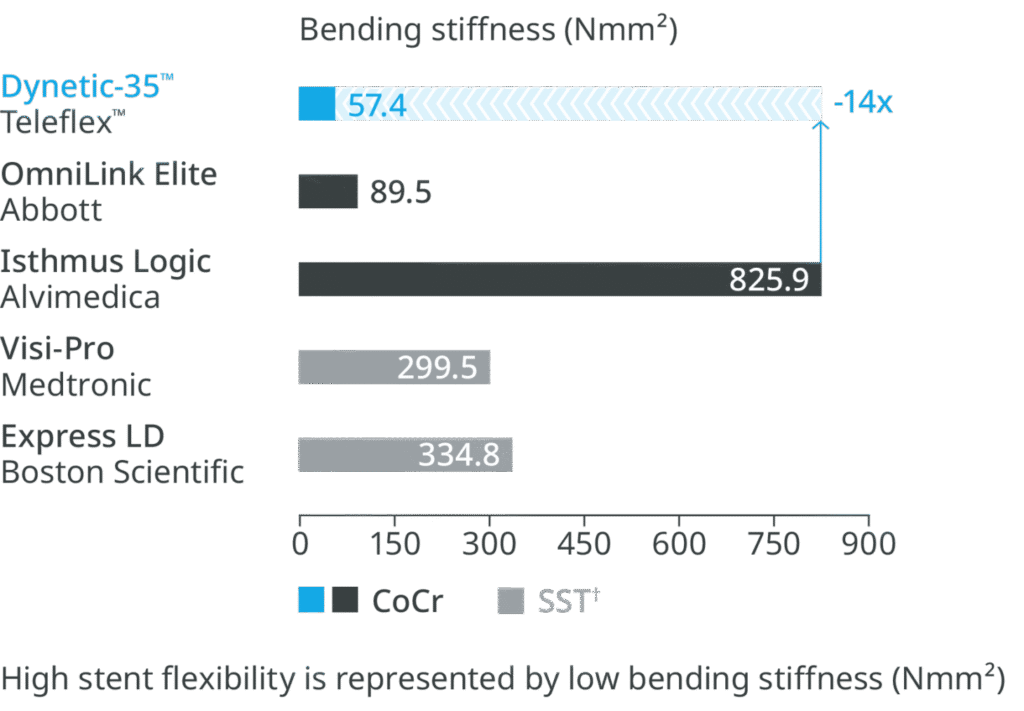
†SST – Stainless Steel

Excellent deliverability
The low profile design with its low crossing profile offers excellent deliverability* compared to leading competitor devices.2,4
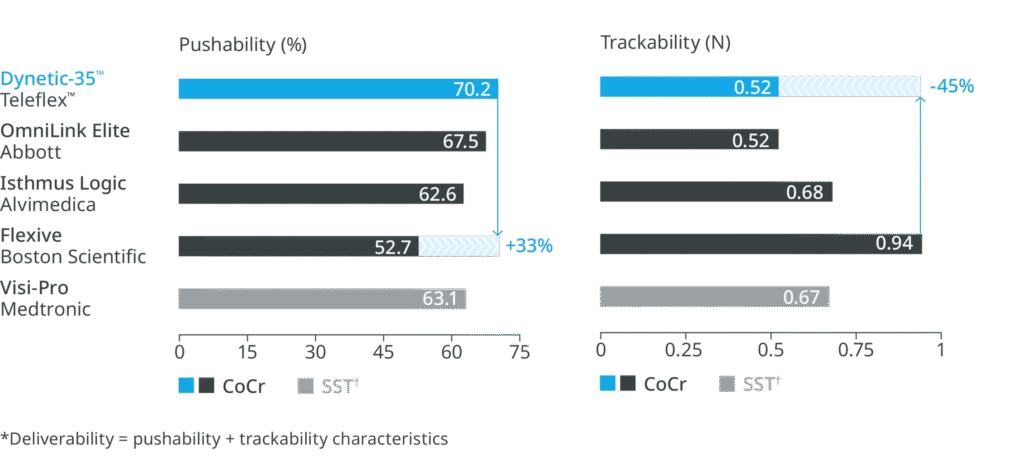
†SST – Stainless Steel
6F compatible in full size range
The thin strut stent design combined with the low profile balloon catheter delivery system offers the full product range in 6F sheath compatibility.4
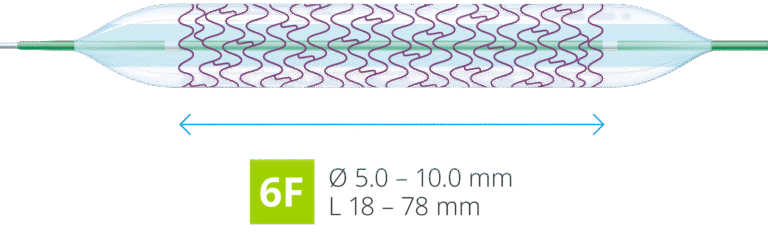
Flexible stent, flexible access options
The only balloon-expandable cobalt chromium iliac stent system with a 170 cm working length3 and full 6F compatibility4, enabling a radial access approach and ambulatory treatment.

Benefits of transradial access:
Patient comfort7
Faster ambulation8

Shorter hospitalization9
- Data on file. IIB(P)13-2019. 10.0 mm stent diameter.
- Data on file. 8.0 mm stent diameter.
- Endovascular Today – Europe Buyer´s Guide 2019, Balloon-Expandable Stents. http://evtoday.com/device-guide/european/152#.
- Data on file.
- Data on file. IIB(P)13-2019. 8.0 mm stent diameter.
- Brodmann M. Dynetic-35 Cobalt chromium balloon-expandable stent for peripheral iliac lesions: 24-month results of the BIONETIC-I Multi-Center study. Presented at: CIRSE, September 16, Barcelona, Spain.
- Rutka JK et al. Comparison of patient comfort after coronary angiography by standard arterial access approaches. Kardiol Pol. 2016;74:68-74.
- Kok MM et al. Patient preference for radial versus femoral vascular access for elective coronary procedures: The PREVAS study. Catheter Cardiovasc Interv. 2018 Jan 1;91(1):17-24.
- Romagnoli E et al. Radial versus femoral randomized investigation in ST-segment elevation acute coronary syndrome: the RIFLE-STEACS (Radial Versus Femoral Randomized Investigation in ST-Elevation Acute Coronary Syndrome) study. J Am Coll Cardiol 2012;60(24):2481–2489.
Teleflex, the Teleflex logo are trademarks or registered trademarks of Teleflex Incorporated or its affiliates in the U.S and/or other countries. All other names are the trademarks or registered trademarks of their respective owners. Refer to this IFU link for a copy of the Instructions for Use and for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use. Not all products may be available in all countries.
© 2025 Teleflex Incorporated. All rights reserved.