All in one solutions. Effective drug delivery. Thin struts.
Achieve more with less.
Peripheral Vascular Intervention
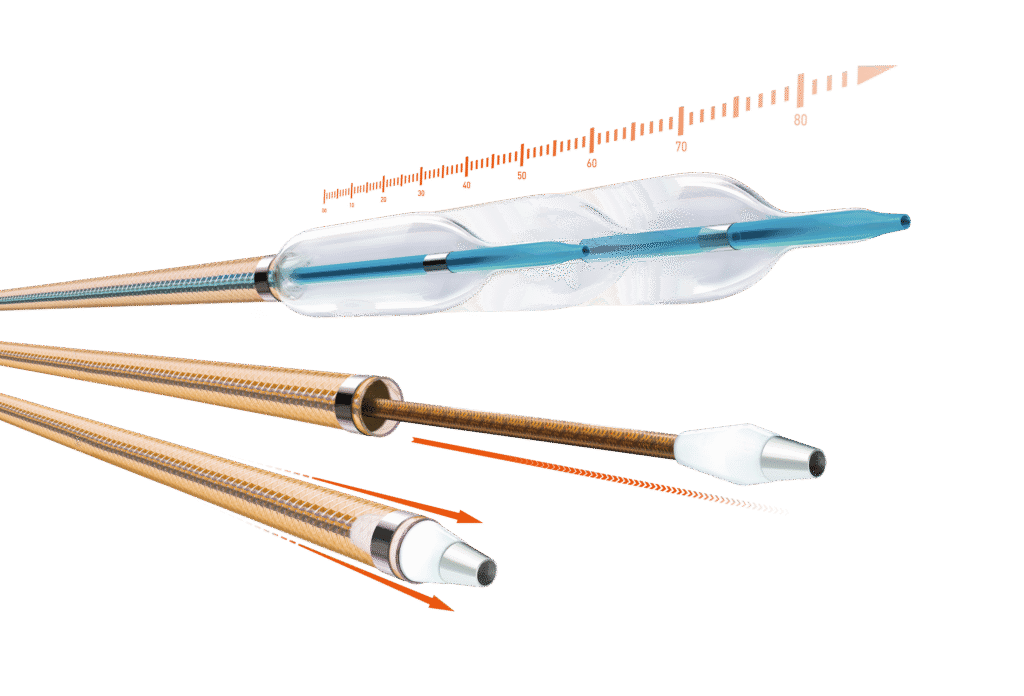
Oscar™
Multifunctional Peripheral Catheter
All-in-one solution to reach, cross and prepare lesions.1
Achieve more with less.

Passeo™– 18 Lux™
Drug Coated Balloon
Excellent results even in challenging patient groups.2
Achieve more with less.
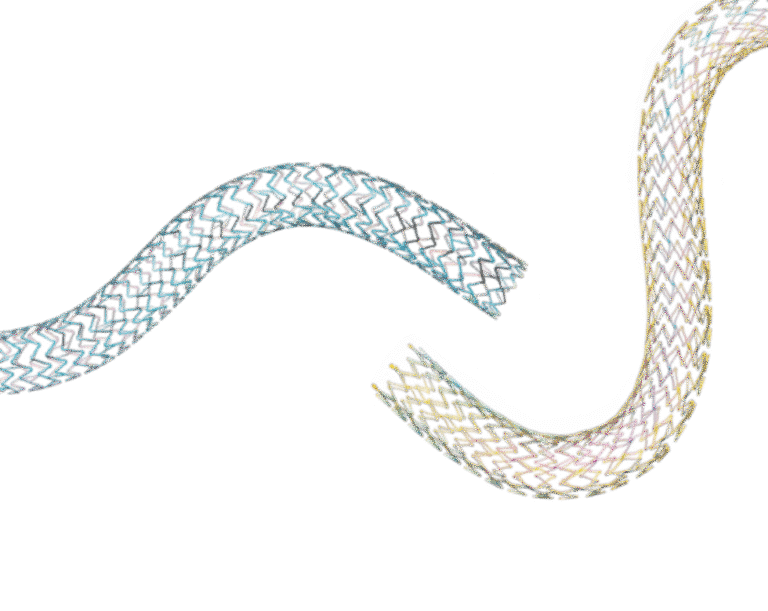
Pulsar™– 18 T3
Self-Expanding Lower Limb Stent
Dynetic™– 35
Balloon-Expandable Iliac Stent
Thin struts – less burden, more flexibility.3-5
Achieve more with less.
Achieve more with less
Oscar™
Peripheral Multifunctional Catheter

One solution. Multiple functions. No compromise
Lesion-specific angioplasty with length-adjustable balloon
User-adjustable guide wire support for accessing and crossing lesions
Passeo™-18 Lux™
Drug Coated Balloon
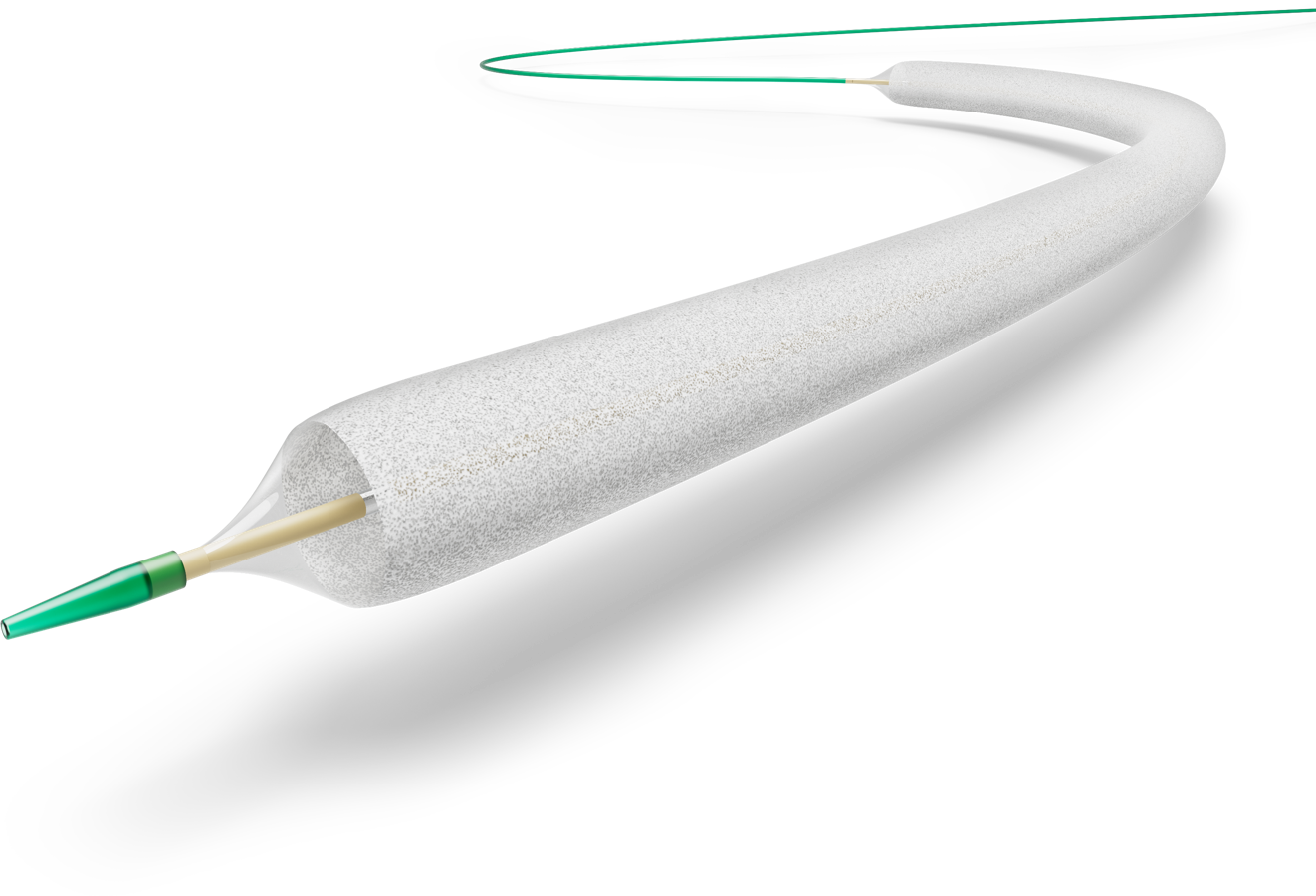

Safe and effective in the treatment of lower limb arteries6,7
Excellent clinical results in complex populations at baseline2
Reduction of drug loss with SafeGuard™ Insertion Aid8
Pulsar™-18 T3
Self-Expanding Lower Limb Stent

Thin struts, low COF
Low profile delivery system
Tri-axial system with braided shaft
Dynetic™-35
Balloon-Expandable Iliac Stent

High radial strength9
Largest size range10
Deliverability in 6F compatibility11
REACT with precision, leave less behind
Every lesion is different, so is the treatment approach.
REACT faster with a full 0.018" solution12

Maximizing outcomes, minimizing burden

Excellent clinical outcomes, proven individually13,14 and in combination15,16
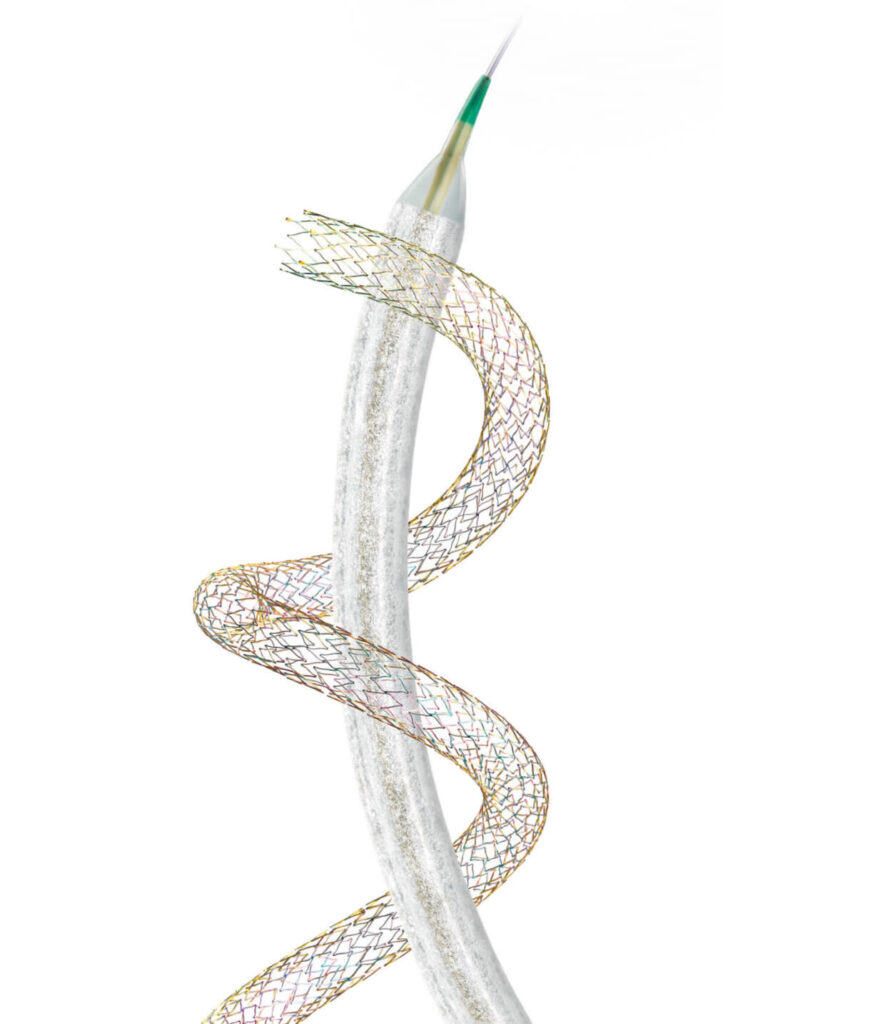
Our comprehensive portfolio
For every step of peripheral vascular intervention — access, crossing, preparation, and treatment — our comprehensive portfolio has you covered.
REFERENCES:
1. per IFU: Indicated for percutaneous transluminal interventions in the peripheral vasculature to provide support during access into and to dilate stenoses in femoral, popliteal and infrapopliteal arteries. The product is also intended for injection of radiopaque contrast media for the purpose of angiography. 2. Tepe G. Paclitaxel-Coated Balloon Angioplasty for the Treatment of Infrainguinal Arteries: 24-Month Outcomes in the Full Cohort of BIOLUX P-III Global Registry. Cardiovasc Intervent Radiol. 2021;44:207-217; 3. Data on file, IIB Bench test report; 4. Koskinas KC et al. Role of endothelial shear stress in stent restenosis and thrombosis, Pathophysiologic mechanisms and implications for clinical translation. JACC 2012;59:1337–49; 5. Foin N et al. Impact of stent strut design in metallic stents and biodegradable scaffolds. Internat J Cardiol. 2014 Dec 20;177(3):800–808; 6. Scheinert D. Paclitaxel Releasing Balloon in femoropopliteal lesions using BTHC excipient: 12-month results from the BIOLUX P-I randomized trial. JEVT. 2015; 22(1): 14-21; 7. Zeller et al. Paclitaxel-Coated Balloon in Infrapopliteal arteries 12-month results from the BIOLUX P-II randomized trial. J Am Coll Cardiol Intv. 2015; 8: 1614-22; 8. Data on file, IIB Bench test report: Investigation of Peripheral Drug Coated Balloons Drug Loss in Introducer Sheath; 9. Data on file, 8.0 mm stent diameter; 10. Endovascular Today – Europe Buyer´s Guide 2019, Balloon-Expandable Stents; 11. Data on file, Design Verification and Validation Result; 12. Ddata on file; 13. Binkert C. BIOLUX P-III Real-world experience with a Paclitaxel-Coated Balloon for the treatment of atherosclerotic infrainguinal arteries: 24-month results of the BIOLUX P-III All Comers Registry in Superficial Femoral Arteries. Presented at: CIRSE 2018. Lisbon, Portugal; 14. Lichtenberg et al. Effectiveness of the Pulsar-18 self-expanding stent with optional drug-coated balloon angioplasty in the treatment of femoropopliteal lesions – the BIOFLEX PEACE All-Comers Registry. Vasa (2019), 1-9. doi_10.10240301- 1526a000785. FTLR and Primary Patency for stent only group; 15. Deloose K. BIOLUX 4EVER: Combining Passeo-18 Lux DCB and Pulsar-18 BMS: 24-month results of full cohort. Presented at: LINC 2018. Leipzig, Germany; 16. Mwipatayi P. DEBAS Study: First-in-man experience of self-expanding nitinol stents combined with drug-coated balloon in the treatment of femoropopliteal occlusive disease. Vascular 2018;26:3–11.
*Excluding central circulatory system and neurovasculature; indicated as per IFU.
Teleflex, the Teleflex logo, Astron, Dynamic, Dynetic, Fortress, Oscar, Passeo, Pulsar, and SafeGuard are trademarks or registered trademarks of Teleflex Incorporated or its affiliates in the U.S and/or other countries. All other names are the trademarks or registered trademarks of their respective owners. Refer to this IFU link for a copy of the Instructions for Use and for a complete listing of the indications, contraindications, warnings and precautions. Information in this material is not a substitute for the product Instructions for Use. Not all products may be available in all countries.
© 2025 Teleflex Incorporated. All rights reserved.
Patient Support Care line:
US & CA | (800) 447-IABP (4227)
International | +1 (978) 250-5177
Customer Service Info:
Toll Free: 866-246-6990
US & CA | 8am-7pm EST / Mon-Fri
Email: cs@teleflex.com